Many individuals have claimed that new drug approvals are declining over time. A recent article by Lanthier et al. (2013) finds the following trends.
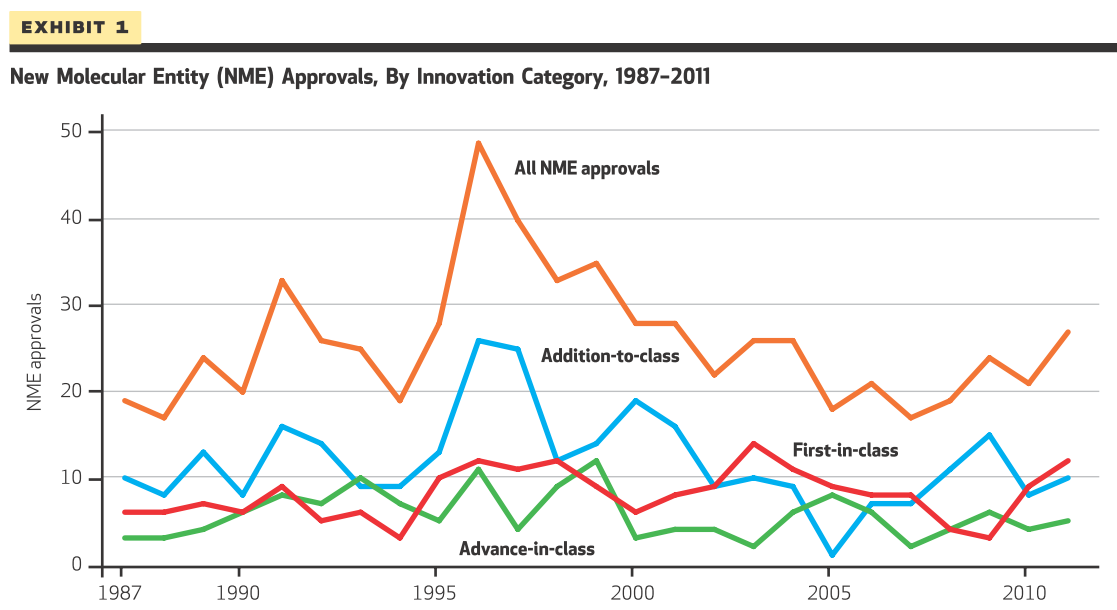
Courtesy of Health Affairs.
Drug approvals seem to be fairly steady over time, except for a decline in addition-to-class drugs. Why is this? The authors hypothesize as follows:
Today we see a trend away from addition-toclass drugs and an increased focus on first-in-class drugs. Although we can only speculate about the causes for this shift, one possible reason deserving of more attention may be the influence of large pharmacy benefit management firms—third-party administrators that process prescription drug claims. These firms, which negotiate discounts with pharmaceutical manufacturers and maintain formularies, may be less willing than other payers to reimburse for highly priced addition-to-class drugs that cannot demonstrate a proven benefit over similar products already on the market. Such decisions could make it less attractive for companies to develop addition-to-class drugs once multiple drugs have established themselves on the market.
Source:
- Michael Lanthier, Kathleen L. Miller, Clark Nardinelli and Janet Woodcock. An Improved Approach To Measuring Drug Innovation Finds Steady Rates Of First-In-Class Pharmaceuticals, 1987–2011. Health Aff August 2013 vol. 32 no. 8 1433-1439
2 Comments