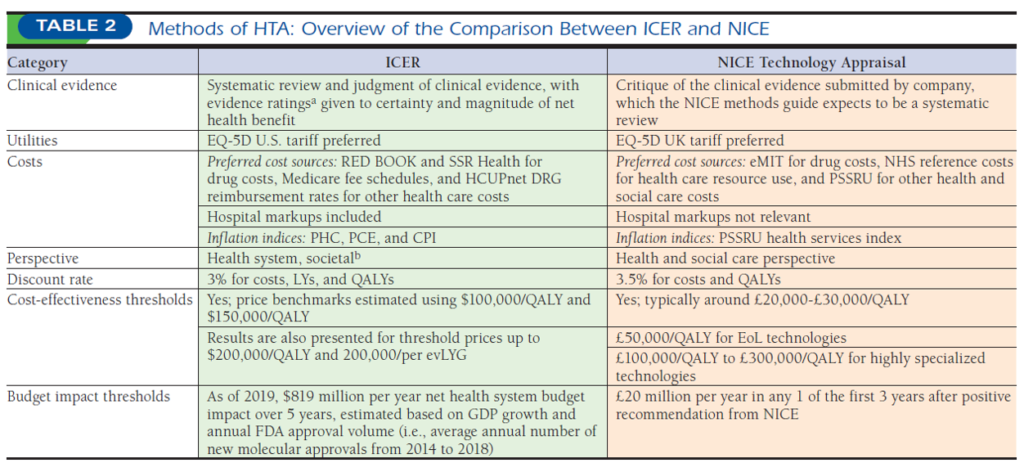
A nice (pun intended) paper by Thokala et al. (2020) compares the Institute for Clinical and Economic Review (ICER) with the National Institute for Health and Care Excellence (NICE) along 4 dimensions: structure, methods, process, and use in decision-making. While ICER and NICE methods are fairly similar, ICER is a non-governmental body without any explicit decision-making power; NICE is a governmental body with decision-making power due to the UK’s single payer system. A more detailed comparison of their methods are below.
The organizations also differ in terms of how much they value innovation versus cost savings.
NICE typically uses a threshold of £20,000-£30,000 per QALY, with a threshold of £50,000 per QALY for technologies that meet end of life criteria,41 and a higher threshold of between £100,000 and £300,000 per QALY when appraising technologies for rare diseases. ICER uses $100,000 and $150,000 per QALY to estimate the value-based price benchmarks (recently renamed as health benefit price benchmarks) and provides incremental results up to $200,000 per QALY and per equal value of life years gained (evLYG).
Timing also differs. NICE evaluations typically take place 12-14 months after UK marketing authorizations whereas ICER starts their reviews 8 months prior to FDA approval.
Recommendations also differ. NICE is limited to only 5 recommendations types: (i) recommended, (ii) optimized, (iii) recommended for use in the Cancer Drugs Fund (cancer appraisals only), (iv) only in research, or (v) not recommended. ICER provides a value-based drug price and recommended discounts as well as their full report, but it’s up to individual payers of how they incorporate that evidence into their price and coverage negotiations.
Source:
- Thokala P, Carlson JJ, Drummond M. HTA’d in the USA: A Comparison of ICER in the United States with NICE in England and Wales. Journal of Managed Care & Specialty Pharmacy. 2020 Sep;26(9):1162-70.