How do you incentivize pharmaceutical companies and researchers to develop new drugs for rare diseases? Drug development is expensive and–by definition–rare diseases have a small market. One solution to this problem is that governments have used orphan drug policies to provide monetary incentives for innovators to research drugs for rare diseases. But which countries have implemented orphan drug policies? And how do these policies differ around the world?
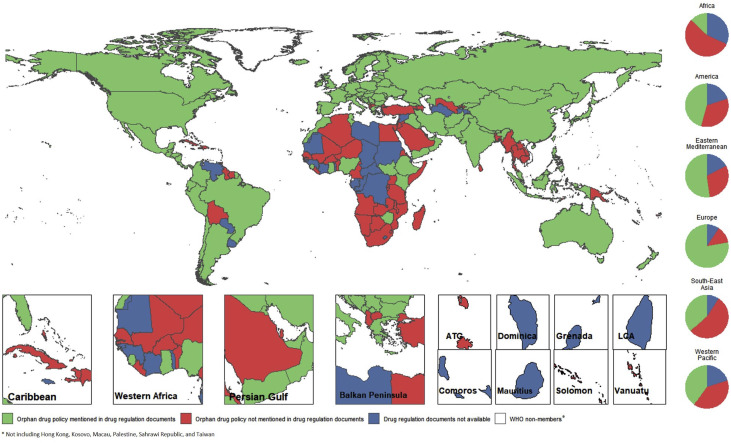
A paper Chan et al. (2020) aims to answer these questions. The authors conducted internet searches, emailed national pharmacovigilance centers, and conducted systematic literature reviews of academic publications. They found that 92 out of 200 countries (46.0%) had documentation on search. The typical disease prevalence threshold for an orphan designation averages 40 to 50 cases per 100 000 population. They also find that:
Of the 37 countries/areas with an established ODP [orphan drug policy] since 2013, 20 (54.1%) were of low- or lower-middle-income, and 10 (27.0%) were of upper-middle income. Nonetheless, at the time of this study, countries/areas with an ODP were statistically wealthier (gross national income per capita = $10 875 vs $3950, P < .001). Orphan drug policies were established in only 19.4% of the 31 low-income countries/areas; indicating a considerable policy gap in these countries/areas.
From the map, one can clearly see that orphan drug policies are more common in wealthier countries.
How do these countries define diseases eligible for orphan drug designation?
…most countries/areas adhered to the European Union definition of low prevalence (0.05%), whereas others followed the number of prevalent cases, such as Australia (<2000), South Korea (<20 000), and the United States (<200 000). Countries/areas such as Chile, Kenya, Peru, and Singapore required the disease severity to be “life threatening” and “‘severely-’ or ‘chronically-’ debilitating.”
Many countries had reduced evidence standards for approval. Many also granted extended patent periods to orphan drugs to incentivize innovation as well as reduced patient cost sharing to incentivize patient access.
Patent protection in the form of market exclusivity was provided for each orphan drug with duration ranging from 5 years (Australia), 7 years (United States), 8 years, or 8.5 years for pediatric orphan drugs (Canada) to 10 years (European Union, Japan, Taiwan)…
China offers tax reductions to reduce value-added tax during the purchasing process of orphan drugs. Patients with rare diseases are able to obtain drugs free or at low prices from government or nongovernment organization subsidies in Colombia and Peru. In Canada and China, orphan drugs can be imported through the Special Access Programme or pathways for orphan drugs that are “highly urgently needed in clinical care,”
Reference:
- Chan AY, Chan VK, Olsson S, Fan M, Jit M, Gong M, Zhang S, Ge M, Pathadka S, Chung CC, Chung BH. Access and Unmet Needs of Orphan Drugs in 194 Countries and 6 Areas: A Global Policy Review With Content Analysis. Value in Health. 2020 Oct 31.