Diversity in clinical trials
Recently, the FDA’s Center for Drug Evaluation and Research’s (CDER’s) released its 2020 Drug Trials Snapshots Summary Report. Part of the report examines the demographics of participants in clinical trials for the 53 drugs that FDA approved in 2020 either as new molecular entities (NMEs) under new drug applications (NDAs) or as new therapeutic biologics under biologics license applications (BLAs). More than 32,000 patients participated in these trials. Were they representative of the overall US population?
In the figure below, I compare the FDA figures against the data from the 2019 US Census population estimates. We see that there are more females patients in clinical trials (56%) compared to the US population. Likely these numbers are skewed by the fact that there were clinical trials for 7 approved breast cancer drugs (with generally >99% of participants being female in the trial) compared to only 3 prostate cancer drugs (with generally 0% of trial participants being female). Unsurprisingly, there are more elderly individuals (30%) compared to the US as a whole (60%).
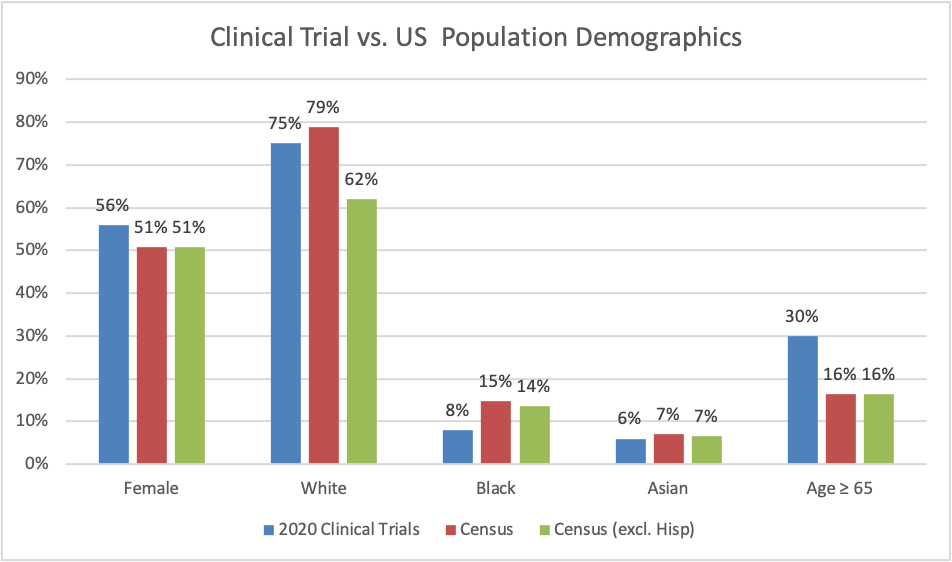
The racial composition is a bit more complex to disentangle. At first glance, it seems like the trials population (with 75% white individuals) has more minority individuals than the U.S. as a whole (with 79% White individuals). However, the 79% figure includes Hispanics as well. Once Hispanics (who make up 18.5% of the US population) are taken out of the Census estimates, the clinical trials appear disproportionately white (75% in trial vs. 62% in the US).
So are the trial populations ignoring minorities? I would say likely no. Although the Census has 79% of the population as White (including Hispanics), this figure rises to 84% among Americans aged 65 and older. Since most of the disease of interest from the clinical trials disproportionately affect the elderly, there are bound to be a higher percentage of White than for the population in general. Additionally, 46% of all clinical trial participants came from outside the US.
Although these data do not provide conclusive evidence, at first glance it appears that the life sciences industry is doing a good job insuring that clinical trials are representative of diverse US populations.
Generic approvals
The FDA’s Office of Generic Drugs also released their 2020 Annual Report. While generics are often ignored in policy debates, they couldn’t be more important. Citing the Association for Accessible Medicines’ 2020 Report, the Office of Generic Drugs writes:
Right now, 9 out of 10 prescriptions in the United States are filled by generic drugs. Generic drugs have saved the health care system $2.2 trillion dollars in the past decade.
In 2020, FDA approved 948 Abbreviated New Drug Applications (ANDAs) including 72 first generics. A visual summary of these approvals is below.
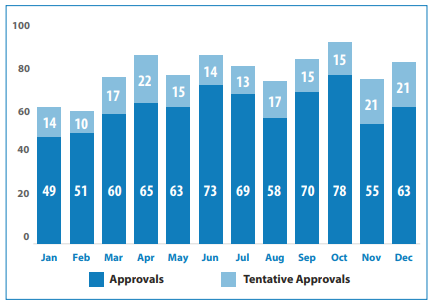
Note: A tentative approval does not allow the applicant to market the generic drug product and postpones the final approval until all patent/exclusivity issues have been resolved.
1 Comment