Last month, the Government Accountability Office released a report on the cost of rare disease. The report is interesting, and especially interesting is Appendix 1 which has a detailed list of studies of the cost of rare disease for about 50 rare diseases. Below are some highlights:
Currently, there are no treatments or cures for most rare diseases. Despite federal incentives for development of products to treat them, there are FDA-approved treatments for only about 5 percent of rare diseases…Even when a drug is available for off-label use, NIH officials explained that the drug would only be available to patients with a rare disease if a physician prescribed it (which they might be unwilling to do in the absence of clear evidence of effectiveness for the patient’s specific situation) and, if prescribed, it might not be covered by insurance.
These incentives mentioned above include the Orphan Drug Act (ODA) ODA provides both tax credits and exclusive marketing rights (beyond those for treatments for non-rare diseases) to incentivize rare disease drug development.
Getting diagnosed for a rare disease is also challenging.
…because there are no treatments for many rare diseases, tests for them might not be considered to affect health outcomes and, therefore, may not be covered by some insurance companies on the grounds that they are “not medically necessary.” For example, whole exome sequencing—a method for identifying variations in the protein-coding regions of genes—has proven useful in testing patients for suspected genetic diseases, but insurance companies do not necessarily cover its use.
Typical cost effectiveness modelling quantifies the value of a diagnostic based on how the new information provided by a diagnostic would change treatment behavior an ultimately health outcomes. However, patients themselves may place a high value on just knowing they have a specific disease. Lakdawalla et al. (2018) calls this “Value of Reducing Uncertainty due to a New Diagnostic”. In part because diagnostic tests are not conducted, the average patient with a rare disease visits 4.2 PCPs, 4.8 specialists, goes to the emergency room 3.7 times and were hospitalized 1.7 times for reasons related to their disease before being diagnosed. Some of the challenges of being diagnosed are shown in the figure below.
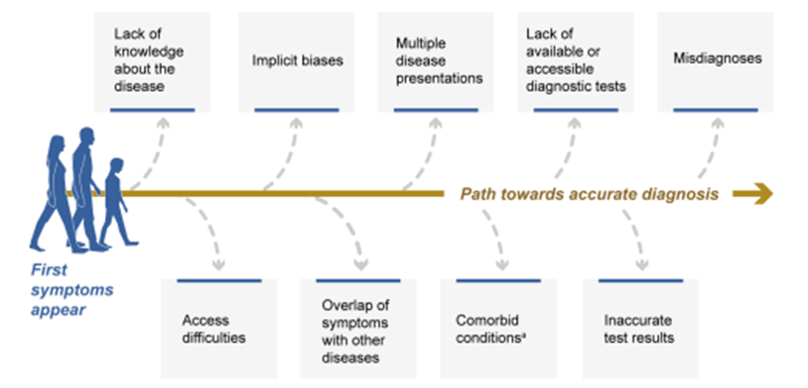
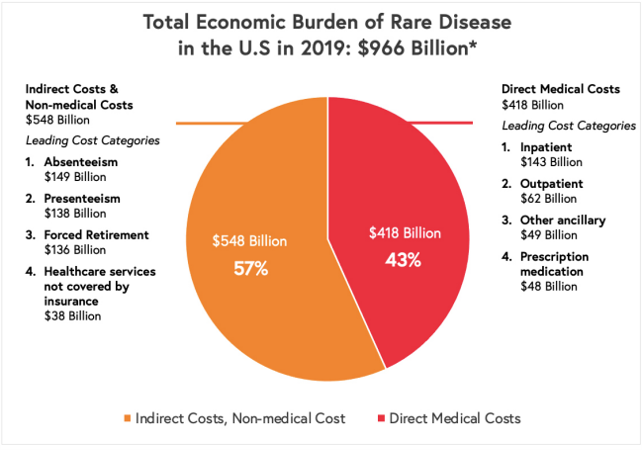
Unsurprisingly, the cost of treating rare diseases is high. One summary or the cost of rare disease across rare diseases is The National Economic Burden of Rare Disease Study, which found that the total annual economic burden of rare diseases was $966 billion.
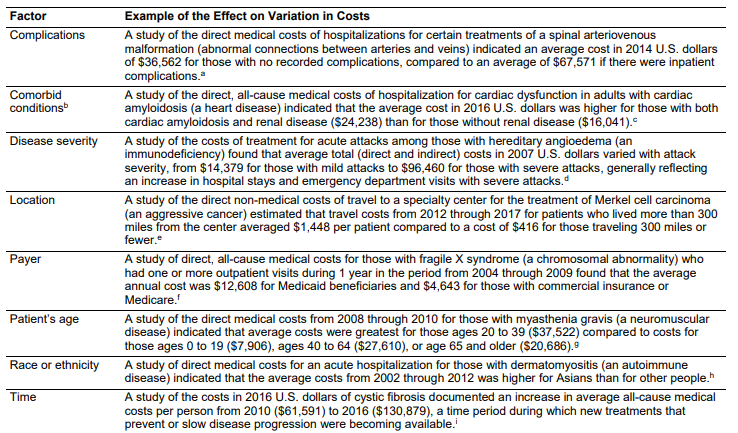
Variation in the cost of rare disease depend on a number of factors, as summarized in the table below.
Do read the whole report for more detail.