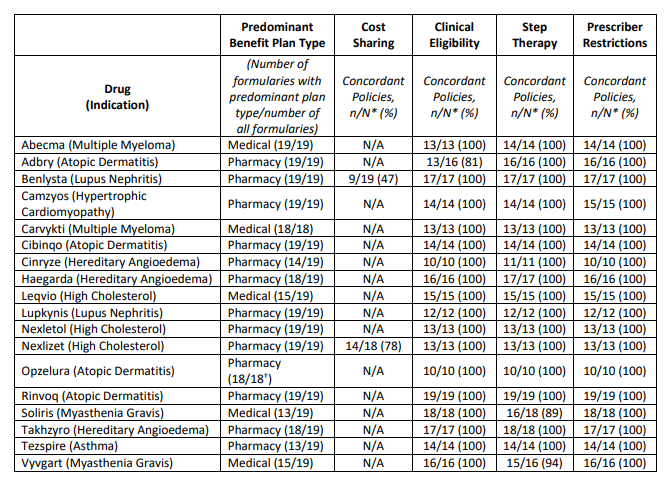
Last month, ICER released their 2023 “Assessment of Barriers to Fair Access” . The report concludes the following regarding 18 drugs evaluated.
ICER defines “fair access” based on the following criteria:
Cost sharing
- Cost sharing based on net price. Patient cost sharing should be based on the net price to the plan sponsor, not the unnegotiated list price.
- No cost for high value therapies. All medications identified by the Internal Revenue Service as high-value therapies should receive pre-deductible coverage within high deductible health plans.
- One low-cost option available in each class. At least one drug in every class should be covered at the lowest relevant cost-sharing level unless all drugs are priced higher than an established fair value threshold.
- Ok to have high cost sharing if no drugs are cost-effective. If all drugs in a class are priced so that there is not a single drug that represents a fair value as determined through value assessment, it is reasonable for payers to have all drugs on a higher costsharing level.
- If all drugs are priced at fair value, formulary placement is acceptable. If all drugs in a class are priced so that they represent a fair value, it remains reasonable for payers to use preferential formulary placement with tiered cost sharing to help achieve lower overall costs.
- Limited cost-sharing if step-through required. As part of economic step therapy, when patients try a lower cost option with a lower cost-sharing level but do not achieve an adequate clinical response, cost sharing for further therapies should also be at the lower cost-sharing level as long as those further therapies are priced fairly according to transparent criteria.
Although ICER list six criteria, only three (#3, #4, and #5) are formally assessed in their report.
Clinical eligibility
- Payers should offer alternatives to prior authorization protocols such as programs that give feedback on prescribing patterns to clinicians or exempt them from prior authorization requirements (“gold carding”) if they demonstrate high fidelity to evidence-based prescribing.
- Payers should document at least once annually that clinical eligibility criteria are based on high quality, up-to date evidence, with input from clinicians with experience in the same or similar clinical specialty.
- Clinical eligibility criteria should be developed with explicit mechanisms that require payer staff to document that they have: (i) considered limitations of evidence due to systemic under-representation of minority populations; and (ii) sought input from clinical experts on whether there are distinctive benefits and harms of treatment that may arise for biological, cultural, or social reasons across different communities; and (iii) confirmed that clinical eligibility criteria have not gone beyond reasonable use of clinical trial inclusion/exclusion criteria to interpret or narrow the FDA label language in a way that disadvantages patients with underlying disabilities unrelated to the condition being treated
- For all drugs: Clinical eligibility criteria that complement the FDA label language may be used to: (i) set standards for diagnosis; and/or • Define indeterminate clinical terms in the FDA label (e.g., “moderate-to-severe”) with explicit reference to clinical guidelines or other standards; and/or (ii) triage patients by clinical acuity when the payer explicitly documents that triage is both reasonable and necessary
- For drugs with prices or price increases that have been deemed reasonable: Except for the three purposes outlined above, clinical eligibility criteria should not deviate from the FDA label language in a manner that would narrow coverage.
- For drugs with prices or price increases that have been deemed reasonable: Documentation that patients meet clinical eligibility criteria should represent a light administrative burden, including acceptance of clinician attestation in lieu of more formal medical record documentation unless documentation is critical to ensure patient safety.
- For drugs with prices or price increases that have been deemed unreasonable: Clinical eligibility criteria may narrow coverage by applying specific eligibility criteria from the pivotal trials used to generate evidence for FDA approval if implemented with reasonable flexibility and supported by robust appeals procedures as described in the implementation criteria.
Step Therapy and Switching
- In order to justify economic step therapy policies extending beyond FDA labeling as appropriate, payers should explicitly affirm or present evidence to document all of the following: • Use of the first-step therapy reduces overall health care spending, not just drug spending
- The first-step therapy is clinically appropriate for all or nearly all patients and does not pose a greater risk of any significant side effect or harm.
- Patients will have a reasonable chance to meet their clinical goals with first-step therapy.
- Failure of the first-step drug and the resulting delay in beginning the second-step agent will not lead to long-term harm for patients.
- Patients are not required to retry a first-line drug with which they have previously had adverse side effects or an inadequate response at a reasonable dose and duration.
- In order to justify required switching policies as appropriate, payers should explicitly affirm or present evidence to document all of the following: (i) use of the required drug reduces overall health care spending. (ii) the required switch therapy is based on the same mechanism of action or presents a comparable risk and side effect profile to the index therapy. (iii) the required switch therapy has the same route of administration or the difference in route of administration will create no significant negative impact on patients due to clinical or socio-economic factors. and (iv) patients are not required to switch to a drug that they have used before at a reasonable dose and duration with inadequate response and/or significant side effects, including earlier use under a different payer
Provider qualifications
- Restrictions of coverage to specialty prescribers are reasonable with one or more of the following justifications: Ii) accurate diagnosis and prescription require specialist training, with the risk that non-specialist clinicians would prescribe the medication for patients who may suffer harm or be unlikely to benefit. (ii) determination of the risks and benefits of treatment for individual patients requires specialist training due to potential for serious side effects of therapy. (iii) dosing, monitoring for side effects, and overall care coordination require specialist training to ensure safe and effective use of the medication.
- Requiring that non-specialist clinicians attest they are caring for the patient in consultation with a relevant specialist is a reasonable option when the condition is frequently treated in primary care settings but some elements of dosing, monitoring for side effects, and/or overall coordination of care would benefit from specialist input for many patients
Fair Access Criteria
- Cost-sharing policies should be presented clearly to consumers prior to health plan selection, allowing all individuals to understand what cost sharing they will face for treatments they are currently taking or are considering.
- Any significant change to formulary or cost sharing structures should not occur mid-cycle unless plan sponsors include this as a qualifying event allowing plan enrollees to switch plans.
- At the point of care, clinicians and patients should be able to rapidly determine the cost-sharing requirements for any treatment along with cost sharing for other alternatives.
- Individuals considering health plan enrollment should be presented with clear information allowing them to understand whether they meet the insurers’ clinical criteria for the treatments they are currently taking. The policies should also set out the rationale behind them and be readily understandable.
- Clinicians and patients should be able to rapidly determine the clinical criteria for any treatment and view the clinical rationale supporting these criteria. The referenced clinical information should be readily available to the prescribing/ordering provider and the public.
- Individuals considering health plan enrollment should be presented with clear information allowing them to understand whether the treatments they currently take or envision taking will be subject to non-medical step therapy or switching policies.
- Clinicians, pharmacists, and patients should be able to rapidly determine the requirements related to step therapy and switching policies and be able to easily view a full justification from the insurer.
- Individuals considering health plan enrollment should be able to easily find information related to coverage criteria, including prescriber qualifications, for drugs that they or family members are currently taking.
- Clinicians and patients should be able to rapidly determine whether there is a restriction on prescribing for any treatment. Insurers should provide ready assistance to primary care clinicians seeking connection with a relevant specialist for consultation as needed
You can read the full report here.