The year 2022 was a good, but perhaps not great year for innovation. According to the FDA’s Center for Drug Evaluation and Research’s (CDER) New Drug Therapy Approvals 2022 report, there were 37 novel drugs approved in 2022. This number is down from the historical rate of approvals between 2013-2021 (43.4 approvals per year) and especially down relative to the last 5 years (51.2 approvals per year).

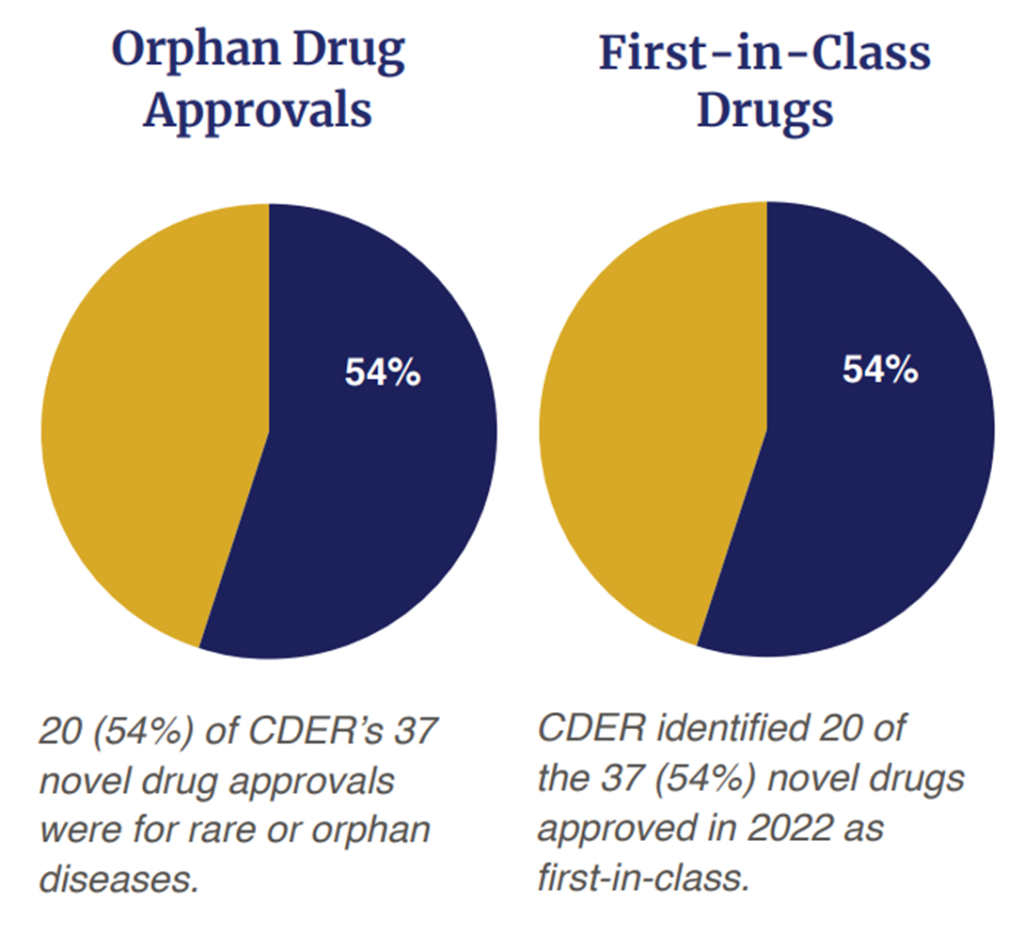
On the other hand, a majority of the drugs (54%, 20 out of 37) were first in class approvals. The era of precision medicine is also upon us as 54% (20 out of 37) were for rare or orphan diseases. Out of the 37 approvals, 32% (n=12) were approved via the Fast Track status; 35% (n=13) were approved with a Breakthrough Therapy designation; 57% (n=21) were approved based on a Priority Review Designation and 16% (n=6) were approved under the Accelerated Approval Program. In short, 65% (n=24) of the 37 drugs approved received some type of expedited review.
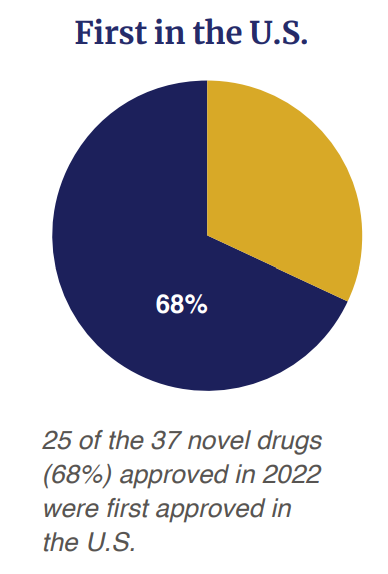
Of the 37 drug approved, 68% (n=25) were first approved in the US before any other country.
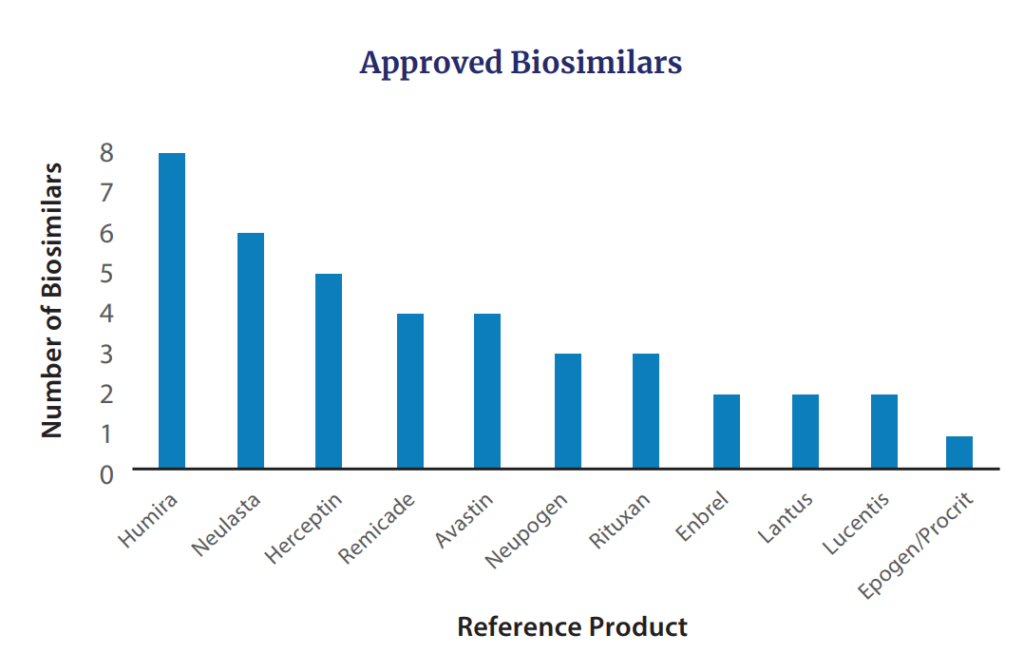
Additionally, in 2022 FDA approved seven biosimilars for products including Avastin (n=2), Humira, Lucentis, Neulasta (n=2), and Neupogen. The cumuulative biosimilars approved by FDA to date are in the figure below.
You can find the full list of product approvals in the FDA report here.