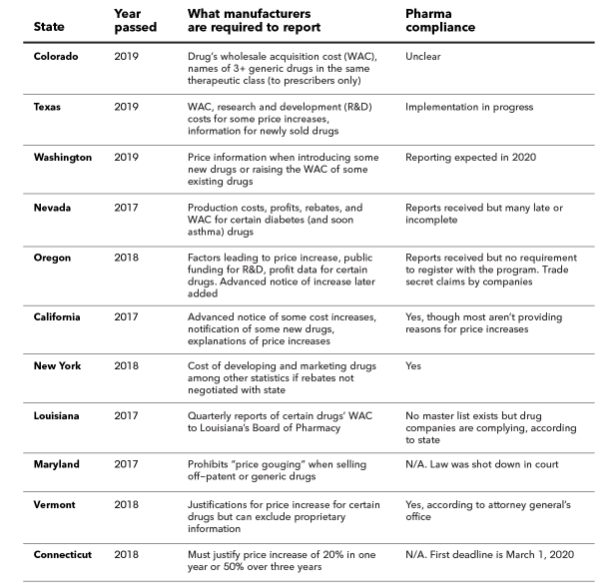
While at the national level, there has been much press about President Trump’s aim of compelling pharmaceutical companies to disclose prices on TV ads (since blocked in the Judiciary), at the state level changes are also afoot. A number of states are making moves to compel pharmaceutical companies to disclose drug prices. Others are compelling pharmaceutical firms to disclose other information including rebates, R&D costs, and profits. Some states mandate this information for all drug classes; others for specific types of drugs. A recent Bloomberg Law article has a nice summary of these laws.
https://news.bloomberglaw.com/health-law-and-business/state-drug-pricing-laws-hampered-by-resistance-lack-of-teeth
The article also states that many of these laws have not been binding. Some manufacturers have complied while others have not.
Only about half of impacted drug manufacturers in Nevada reported on time in 2019, with some citing staff turnover or unfamiliarity with the law, said Scott Jones, who previously worked with the drug price transparency program in the state’s Department of Health and Human Services.
California regulators sent 17 penalty notices for late reports totaling nearly $11 million in fines, according to Jan. 22 data released to Bloomberg Law under the state’s public records law. Five companies paid in full, while others reached settlements to pay reduced amounts or nothing, or are still appealing.
The price transparency laws are an evolving area, and are a policy area that may be affected by the 2020 Presidential Election.
I like how clear cut/plain language Connecticut’s policy is. It will be interesting how compliance looks there and how many drugs are held to a 19% increase (just below the cap).
Max